In the contemporary digital age, the role of minerals in the manufacturing of electronics cannot be understated. These minerals, often referred to as ‘technology metals‘, are critical components in a variety of electronic devices ranging from smartphones and computers to advanced machinery and renewable energy technologies. They are integral to the functionality of these devices, with their unique properties enabling everything from energy efficiency and durability to power storage and semiconducting capabilities. Technological advancements have surged the global demand for these minerals, with the market size expected to reach $10.61 billion by 2027, according to a report by Grand View Research.
However, the extraction and processing of these minerals pose significant environmental and ethical challenges. Many of these minerals are sourced from regions associated with political instability, human rights abuses, and environmental degradation. For instance, coltan, a mineral used in capacitors that power a range of electronic devices, is primarily sourced from the Democratic Republic of Congo, a region plagued with issues of illegal mining and conflict. Moreover, the mining process itself is energy-intensive and contributes to substantial CO2 emissions.
Copper
Copper’s key properties make it an ideal material for various electrical applications. First, its use in circuit boards is significant due to its excellent conductivity, reducing the risk of overheating. Furthermore, the metal’s flexibility and strength make it a preferred choice for cables, ensuring durability and longevity. Lastly, considering its resistance to corrosion, copper is commonly employed in wiring across multiple industries. Hence, these uses substantiate the vital role of copper in electrical engineering.
Used in Circuit Boards
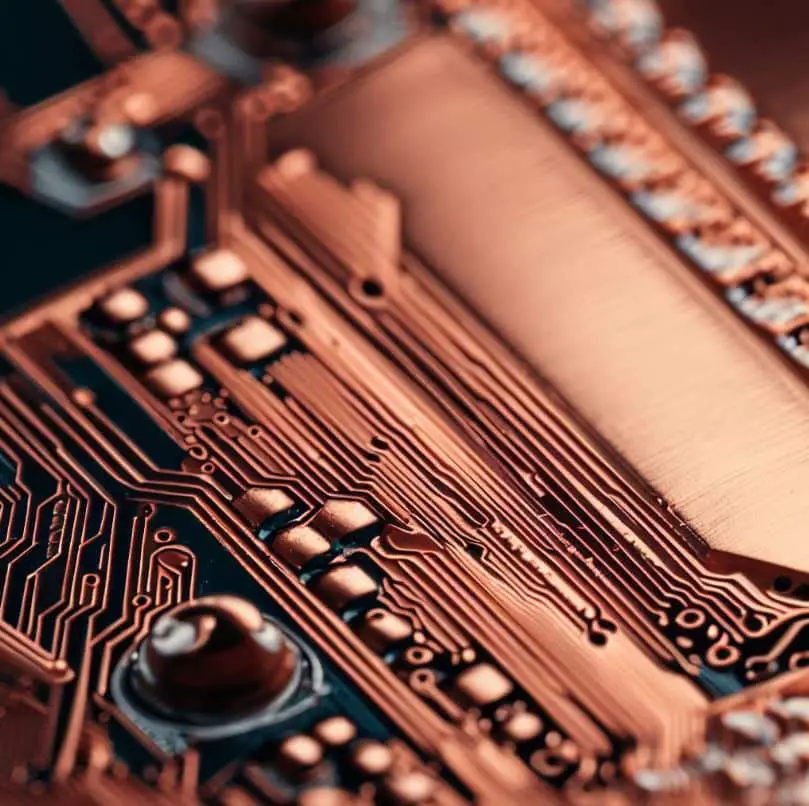
Renowned for its excellent electrical conductivity, copper is the primary material used in the fabrication of circuit boards. The integration of copper in these components allows for more efficient transmission of electrical currents, thereby optimizing the functionality of the electronic devices in which they are installed.
In fact, according to the Copper Development Association Inc., approximately 28% of the global copper output is utilized in the electronics industry, a large proportion of which is dedicated to circuit board production. This is because copper lines, which are often etched onto the circuit board, serve as the conduit for the flow of electrical signals. Their efficiency in conducting electricity ensures that the device operates optimally with minimal energy waste. Consequently, the use of copper in circuit boards has become an industry standard, contributing significantly to advancements in electronic technologies.
Used in Cables
Transitioning from the canvas of its historical and mineralogical context, let’s delve into the practical applications of copper. In particular, let’s focus on its use in cables.
Copper is, in fact, the most widely used material for the manufacturing of electrical cables. This is due to its excellent electrical conductivity. And guess what? Its flexibility and strength also make it an optimal choice in the production of cables.
A 2017 report by the International Copper Association revealed that about 60% of total copper use is for conducting electricity. It predominantly finds its place in the manufacturing of power cables, telecommunication cables, and wiring.
Moreover, copper cables are significantly reliable and require less maintenance. They are also highly resistant to corrosion, which greatly extends their lifespan. It’s no wonder, then, that copper has become the industry standard in cable production.
So, what’s the catch? Well, copper is not only versatile and reliable but also recyclable.
Used in Wiring
Shifting gears to a more focused discussion, the use of copper in wiring proves to be instrumental. In fact, copper is a popular choice due to its high electrical conductivity and flexibility. Furthermore, copper wiring is less prone to corrosion, ensuring longevity and reliability.
Consider this, copper has been widely used in wiring since the telegraph in the 1820s. Its ability to transmit signals efficiently and effectively is unparalleled. In terms of electrical power transmission, copper wires are preferred because of their ability to resist heat.
And that’s not all, copper wiring is also crucial in several industries such as telecommunications, electronics, and construction. For example, in the construction industry, copper wires are the standard for residential and commercial wiring systems.
In conclusion, the role of copper in wiring is undeniable, as it proves to be a versatile, reliable, and efficient material. This assertion underscores the enduring relevance of copper in today’s technological landscape.
Nickel
Nickel’s significant application in batteries, particularly lithium-ion cells, contributes to their efficiency and sustainability. Furthermore, Nickel plays a crucial role in the manufacture of magnets due to its ferromagnetic properties, enhancing their performance. Consequently, the future of Nickel in the electronics industry seems promising given its versatile attributes. Its increased use in innovative electronics production suggests a pivotal role in addressing future challenges.
Oman granted block 21 with an area of 1444 sq kilometers to the British company Knights Bay. The production target will be 111,000 tonnes per year of nickel ore and 5,100 tonnes per year of cobalt at full production.
Used in Batteries
Shifting from copper’s multifaceted utility, attention now turns to another vital metal, nickel. As with copper, nickel’s importance in technology cannot be overstated, particularly in the realm of batteries. In fact, the majority of lithium-ion batteries, the most popular battery technology, use nickel as a key component.
Nickel’s high energy density and stability at high temperatures make it an optimal choice for battery manufacturing. For example, nickel-cadmium (NiCd) and Nickel-Metal Hydride (NiMH) batteries are renowned for their long cycle life and robustness under strenuous conditions. It is also noteworthy to mention the critical role of nickel in the production of Lithium Nickel Manganese Cobalt Oxide (NMC) and Lithium Nickel Cobalt Aluminum Oxide (NCA) batteries, which are extensively used in electric vehicles and portable devices.
Used in Magnets
Now, shift your attention to another versatile metal, nickel. An equally significant player in the magnetic field, nickel’s magnetic properties are widely exploited in various applications.
Primarily, nickel finds robust usage in the manufacture of permanent magnets. These magnets generate their own persistent magnetic field, unlike temporary magnets. Alloys of nickel, such as Alnico, an iron-aluminium-nickel-cobalt alloy, exhibit strong magnetic properties.
Moreover, nickel plating is often used to enhance the magnetic properties of other materials. For example, nickel-iron thin films serve as an important component in magnetoresistive random-access memory (MRAM) devices.
To illustrate, nickel’s magnetic features contribute to the development of advanced technologies, such as Magnetic Resonance Imaging (MRI) scanners. It’s worth noting that nickel’s magnetic properties are temperature-dependent, becoming more magnetic as temperature decreases.
Evidently, nickel’s magnetic properties hold significant value in diverse applications, marking its importance in the magnetic field.
Future of Nickel in the Electronics Industry
Now, let’s pivot to the future of nickel in the electronics industry. It is projected that nickel, with its unique properties, will play a crucial role in the future of electronics. As the industry continues to innovate, the demand for high-performance materials like nickel is expected to rise. For instance, the growing trend of miniaturization in electronics requires materials with excellent conductivity and corrosion resistance, qualities inherent in nickel.
Furthermore, the development of advanced technologies such as artificial intelligence and 5G networks will necessitate the use of nickel due to its magnetic properties. This makes nickel vital for producing components that can efficiently store and transmit data.
The World Steel Association anticipates that the demand for nickel in electronics will double by 2050. Therefore, in the coming years, it is expected that nickel’s importance in the electronics industry will only continue to grow, underlining the need for more sustainable and efficient methods of nickel extraction and recycling.
Aluminum
Aluminum’s versatility extends to its applications in heat sinks, where it’s used to disperse heat generated by electronics. Its exceptional thermal conductivity and lightweight nature make it a preferred choice in electronics manufacturing. The recyclability of aluminum also contributes to its desirability in the electronics industry. It offers an eco-friendly option, with recycled aluminum using only 5% of the energy required for primary production. Therefore, recycling and reusing aluminum is a sustainable practice that deserves thorough exploration.
Used in Heat Sinks
Moving from the resilient nature of nickel, there’s another metal with its own set of fascinating properties. This brings the discussion to Aluminum, particularly its use in heat sinks.
Aluminum is universally renowned for its exceptional heat conductivity capability. This characteristic makes it an ideal material for heat sinks in a wide range of electronic devices. Heat sinks are crucial as they help to dissipate the excessive heat produced by electronic components, thereby preventing overheating and subsequent damage. Aluminum provides an efficient, cost-effective solution in this regard due to its abundance and easy availability.
Thermal resistance is another property that makes aluminum a preferred choice for heat sinks. It refers to the material’s ability to resist the flow of heat. Aluminum exhibits a low thermal resistance, allowing heat to flow freely, thus ensuring optimal performance of electronic devices. Furthermore, the lightweight nature of aluminum adds to its appeal, minimizing the overall weight of the electronic devices.
Use of Aluminum in Electronics Manufacturing
Moving on from nickel, let’s delve into another fascinating metal, aluminum. This lightweight and durable material plays a crucial role in electronics manufacturing, offering both functional and environmental benefits.
Firstly, aluminum is renowned for its excellent conductivity: it is second only to copper in terms of its ability to conduct electricity. This makes it a preferred choice for manufacturers of electronic devices, from smartphones to televisions. Secondly, aluminum’s ability to withstand high temperatures makes it an ideal material for use in electronic components that generate significant heat.
Furthermore, the use of aluminum in electronics manufacturing is not limited to its functional attributes. It also provides aesthetic features, being easily moldable and possessing a clean, modern look. This makes aluminum a popular choice for items that are not only functional but also appealing to the eye, such as laptops and tablets.
In conclusion, aluminum is a vital player in the electronics manufacturing industry due to its unique blend of functional features and aesthetic appeal.
Recycling and Reusing Aluminum in Electronics
Shifting gears from nickel, let’s dive into another vital metal, aluminum. Aluminum offers remarkable flexibility in electronics manufacturing due to its malleability and compatibility with various alloys. But did you know, it’s also one of the most commonly recycled materials in the world?
Indeed, aluminum recycling and reuse in electronics manufacturing play a crucial role in reducing environmental impact. According to the Aluminum Association, nearly 75% of all aluminum ever produced is still in use today, thanks to recycling efforts. This is significant as recycling aluminum requires only 5% of the energy compared to primary production, reducing emissions substantially.
Moreover, recycling aluminum mitigates the need for mining new material, thereby protecting the Earth’s crust from further damage. Not forgetting the economic benefits, the aluminum recycling industry contributes over $800 million to the U.S. economy annually.
In summary, the recycling and reusing of aluminum in electronics manufacturing presents significant environmental, economic, and energy-saving benefits.
Tin
Tin, a versatile metal, plays a pivotal role in the realm of soldering. This application, extensively used in electronics, contributes significantly to tin’s global demand. The uses of tin in electronics extend beyond soldering, encompassing various components and devices. It is, therefore, crucial to explore these uses in depth. Moreover, the global production of tin also merits attention, considering the metal’s widespread usage. This exploration will include top producing countries, the production process, and how the market influences the industry.
Used in Soldering
Moving on from the versatile element of Aluminum, let’s delve into the world of another fascinating metal – Tin. Shifting the lens towards soldering, an area where tin plays a paramount role.
Tin, with its low melting point of 231.93°C, forms the base element in solder, a material widely used to join metals. Solder, typically a blend of tin and lead, provides a secure connection between electrical components. The ability to conduct electricity makes tin indispensable in the realm of soldering.
In the past, a 60/40 tin-lead solder was standard. However, due to health and environmental concerns about lead, manufacturers have moved towards lead-free solders. These typically contain a high percentage of tin, often combined with silver or copper.
It’s important to note that the quality of a solder joint is critical in electronics. A poorly soldered joint can lead to circuit failures, making the role of tin even more significant.
Uses of Tin in electronics

The primary application of tin in electronics lies in soldering. Tin is the chief component of solder because it provides the necessary low melting point, allowing the solder to flow and create a reliable, electrical connection.
Furthermore, the anti-corrosive properties of tin make it an ideal choice for coating other metals. The coating is used extensively in the electronics industry to prevent oxidation that can damage electronic components.
Moreover, tin is used in the production of capacitors, resistors, and inductors. These components are essential for regulating the flow of electricity in electronic devices.
The growing electronics industry is expected to drive the demand for tin in the future. The use of tin in electronics illustrates its vital role in modern technology.
Global Production of Tin
Moving on from the flexible world of aluminum, let’s venture into the domain of tin. Just as fascinating, tin displays a different set of properties and uses.
Focusing on the global production of tin, it is interesting to note that this element is not evenly distributed. According to the US Geological Survey, China holds the title of the world’s largest producer of tin, contributing to nearly 30% of the global yield in 2020. In the same year, Indonesia followed closely, with just under 20% of the total global production.
However, it’s essential to acknowledge that the global production of tin has been experiencing a steady decline over the past decade. In fact, the International Tin Association reports a decrease of approximately 2% per annum. As tin is a vital component in electronics and soldering, this downward trend could have significant implications for these industries. This highlights the need for sustainable mining practices and efficient use of this valuable resource.
Silver
Silver’s application in soldering demonstrates its versatility and critical role in various industries. Utilized for its excellent thermal and electrical conductivity, silver’s use in electronics is next to consider. Endowed with unique chemical properties, such as the highest electrical conductivity among all elements, silver’s functionalities extend beyond surface value. The following sections will delve deeper into these subtopics, shedding light on silver’s multifaceted nature.
Used in Soldering
Bidding adieu to tin, let’s dive into the world of silver, a metal with some intriguing properties. One of the most common applications of silver is in soldering processes. Soldering, an essential process in electronics manufacturing, often incorporates silver due to its high thermal and electrical conductivity.
In addition, silver-based solder provides numerous benefits over traditional tin-lead solder. It yields a mechanically stronger joint and offers enhanced fatigue resistance. Furthermore, it is less prone to the formation of ‘tin whiskers’, a common problem associated with tin-lead solders that can lead to electrical failure.
Research suggests that the addition of silver into solder alloys can significantly improve wetting properties, leading to better formation of solder joints. However, the cost of silver often limits its use to high-reliability or high-performance applications.
In conclusion, the use of silver in soldering enhances the overall performance of the solder joint, despite being a costlier option.
Silver in Electronics
Shifting gears from tin, let’s delve into the world of silver, an equally precious and versatile element.
The application of silver in electronics is particularly notable due to its superior electrical conductivity. Among all elements, silver has the highest electrical conductivity, making it an essential component in several electronic devices. In fact, 36% of silver consumption in the United States stems from the electrical and electronics industry.
For instance, silver is used in multi-layer ceramic capacitors (MLCCs), which are integral parts of modern electronic devices like mobile phones and computers. Additionally, silver’s ability to endure extreme temperature fluctuations makes it ideal for use in automobile electronics.
Its role doesn’t stop there. Silver is also a crucial ingredient in printed circuit boards (PCBs), due to its excellent solderability and efficient heat transfer.
In conclusion, the superior electrical properties of silver make it an indispensable component in the realm of electronics.
Silver’s Chemical Properties
Shifting focus from the versatility of tin, let’s delve into another equally fascinating element – silver.
Exhibiting a unique blend of chemical properties, silver is a standout in the periodic table. With an atomic number of 47, silver is a transition metal that sits in the same group as copper and gold. It’s renowned for its high thermal and electrical conductivity.
Remember this: silver is an incredibly reactive metal. It tarnishes when exposed to ozone, hydrogen sulfide, or air-containing sulfur due to the formation of silver sulfide. Despite this, it’s relatively stable in pure air and water because it forms a protective oxide layer.
Take note, it’s not only the reactivity of silver that’s intriguing. It’s also interesting to note that silver has the highest electrical conductivity of all elements, a property that makes it invaluable in various technological applications.
Without a doubt, silver’s diverse chemical properties make it an element of significant interest and utility.
Iron
Iron’s versatility extends to its application in connectors and relays, where its durability and conductivity play a crucial role. The electrical conductivity of iron, although not as high as that of copper or silver, ensures efficient energy transmission. This property is particularly significant in electronic components, where iron, in various alloy forms, contributes to the performance and longevity of devices. Indeed, the importance of iron in these components cannot be overstated.
Used in Connectors and Relays
Stepping away from the lustrous charm of silver, let’s delve into the robust world of iron. Used extensively in connectors and relays, iron’s inherent properties make it an ideal choice for these applications.
Iron, a ferromagnetic material, is often used in electrical relays due to its ability to maintain a magnetic field after removing the magnetizing force. This property, called remanence, is what makes iron an invaluable component in relays.
Furthermore, iron’s robust nature and high melting point make it the preferred choice for use in connectors. These connectors, often used in power supplies and electronic equipment, require materials that can withstand high temperatures and pressure.
In summary, due to its ferromagnetic properties and robust nature, iron finds extensive use in connectors and relays in electronics. This application, among others, highlights the significance of iron in various industries. Now, it’s time to turn attention to the electrical conductivity of iron.
Iron’s Electrical Conductivity
Swapping our attention from the lustrous white metal, silver, let’s dive into the world of another essential element, iron. Unlike the superior electrical conductivity of silver, iron has a relatively lower electrical conductivity. Iron conducts electricity at a rate of approximately 1×10^7 S/m, which is significantly lower than that of silver.
However, the conductivity of iron changes with temperature, increasing as the temperature decreases. This can be attributed to the fact that, at lower temperatures, the scattering of conduction electrons by lattice vibrations is reduced, increasing the electrical conductivity. Iron’s electrical conductivity is also influenced by the presence of impurities and alloying elements. For instance, the addition of carbon, a common alloying element in steel, tends to decrease the electrical conductivity of iron.
This characteristic of iron, along with its abundance and relatively low cost, has led to its use in a variety of electrical applications. Even though its conductivity is lower than that of silver, iron’s unique properties make it indispensable in the electrical and electronics industry.
Iron in Electronic Components
Shifting from the sheen of silver to the sturdiness of iron, iron holds immense significance in the world of electronics. Iron finds its utilization in many electronic components, including transformers, inductors, and magnetic storage devices.
Diving deeper into the subject, iron cores are commonly used in transformers due to their high magnetic permeability. This property of iron aids in efficiently transferring alternating current from one coil to another.
Further down the line, iron comes into play in inductors, which are electronic components that store energy in their magnetic field. The higher the permeability of the core material, the better the performance of the inductor. Iron, with its high permeability, fits the bill perfectly.
Moreover, iron is also employed in magnetic storage devices such as hard disks. These devices use tiny magnetized iron particles to store data. The use of iron, therefore, underscores its indispensable role in the electronics industry.
Manganese
Manganese is a fundamental element in battery production, particularly in alkaline batteries and lithium-ion batteries. Manganese in batteries serves as an essential component in the cathode material, enhancing energy density and stability. However, the mining of manganese raises significant environmental concerns. The environmental impact of manganese mining includes habitat destruction, water pollution, and heavy metal contamination, necessitating the exploration of sustainable mining practices and alternative materials.
Used in Batteries

Shifting gears from iron, let’s delve into the fascinating world of manganese. Primarily, manganese plays a pivotal role in the production of batteries. To be specific, lithium-ion batteries, the power source for many electronic devices and electric vehicles, utilize manganese in their chemistry.
Manganese compounds, specifically lithium nickel manganese cobalt oxide (NMC), are used in the cathodes of these batteries. The NMC cathodes have a high energy density and long lifespan, making them ideal for use in power tools, electric vehicles, and large-scale energy storage systems.
Moreover, manganese is also utilized in alkaline batteries and zinc-carbon batteries. In these batteries, manganese dioxide acts as the cathode material, which reacts with the zinc anode to produce electricity.
In summary, the usage of manganese in batteries is widespread, from small-scale electronic devices to large-scale energy storage systems, demonstrating its crucial importance in the energy sector.
Manganese in Batteries
Stepping away from the realm of iron, the focus now shifts to its periodic table neighbor: manganese. As a key element for batteries, manganese holds a pivotal role in energy storage. The spotlight here is on manganese in batteries.
Manganese’s unique properties make it a prime candidate for battery development. Its high energy density and thermal stability make it a crucial component in Lithium-ion batteries. The manganese-based cathode material, known as Lithium Manganese Oxide (LMO), exhibits a high discharge voltage, which is instrumental in providing power to electric vehicles and portable devices alike.
Not to mention, manganese’s abundance and relatively low cost also contribute to its widespread use. For instance, the manganese dioxide-zinc alkaline battery, a common household battery, utilizes the electrochemical properties of manganese dioxide. These batteries are known for their longevity and reliability, making them a popular choice for everyday devices. In essence, manganese batteries are a cornerstone of modern technology.
Environmental Impact of Manganese Mining
Moving from iron, let’s shift gears and delve into the realm of manganese, another important metal. This section will specifically scrutinize the environmental impact of Manganese mining.
Manganese mining, akin to other types of mining, carries significant environmental implications. Notably, the extraction process often leads to deforestation and habitat degradation. Moreover, the associated waste materials and toxins precipitate soil and water pollution, thereby disrupting local ecosystems. A report by the Environmental Protection Agency states that manganese mining can also lead to air pollution with manganese particles, posing a risk to human health. Strikingly, a study published in the Journal of Environmental Sciences in 2016, revealed that manganese mines released over 14,000 tons of particulate matter into the atmosphere.
While manganese is indispensable for various industries, including battery manufacturing, its mining poses serious environmental challenges that need immediate and effective mitigation strategies.
Zinc
Zinc’s versatility extends to its utility in antennas, where it enhances signal strength. Furthermore, the incorporation of zinc alloys in electronic components contributes to improved durability and performance. The technological progression in zinc processing, driven by continuous innovation, has optimized its applications in electronics. This expands the efficiency and longevity of electronic devices, a testament to zinc’s indispensable role in the electronics industry.
Used in Antennas
Imagine the potential of a material that not only protects against corrosion but also enhances signal transmission. That’s the magic of zinc in antenna manufacturing. The use of zinc, particularly in the form of galvanized steel, significantly improves the quality of signal reception. As an essential component of antenna design, zinc provides superior resistance to weathering and corrosion, ensuring a longer lifespan for the antenna.
Furthermore, due to the natural electrical conductivity of zinc, it can boost the antenna’s signal transmission capabilities. This feature is particularly relevant in the era of digital and satellite communications where signal clarity is paramount. The application of zinc has been instrumental in the development of high-performance antennas. New research continues to uncover the impressive capabilities of zinc in this field, reinforcing its vital role in the ever-evolving landscape of telecommunications.
In conclusion, this section has shown that the properties of zinc make it a valuable asset in antenna manufacturing, with benefits ranging from robustness to enhanced signal reception.
Zinc Alloys in Electronic Components
Leaping from the properties of manganese, let’s dive into the significance of another key element: Zinc. The focus here is on the role of zinc alloys in electronic components.
In the realm of electronics, zinc alloys have become indispensable. These alloys are primarily used in precision components such as connectors, switches, and housing for various devices. This is attributed to their exceptional properties including high electrical conductivity, corrosion resistance, and high strength-to-weight ratio. A prime example is the utilization of zinc-aluminum alloy, known as Zamak, in the production of connectors and electromagnetic shielding.
Moreover, the characteristics of zinc alloys such as low melting point, good fluidity, and easy casting make them ideal for manufacturing complex electronic parts. These properties also make zinc alloys cost-effective, thereby promoting their widespread use in the electronics industry.
This discussion about zinc alloys highlights their significance, not only in the electronic components but also in evolving technological advancements.
Technological advancements in zinc processing for electronics
In recent years, advancements have been made in zinc recovery and reuse from electronic waste. This has been driven by the growing need for sustainable and efficient methods of e-waste management. For instance, hydrometallurgical processing techniques have been developed that can efficiently extract zinc from electronic waste. This process involves the use of solvents to dissolve metals, allowing for their subsequent recovery.
Moreover, the development of nanostructured zinc has revolutionized its applications in electronics. Nanostructured zinc, due to its enhanced electrical properties and high surface area to volume ratio, has found applications in supercapacitors, piezoelectric devices, and photovoltaic cells.
These advancements in zinc processing technologies have not only improved the efficiency and performance of electronic devices but have also contributed significantly to the reduction of electronic waste and the promotion of resource sustainability.
Gold
The versatility of gold extends beyond its aesthetic appeal. In the world of electronics, gold is integral in the production of connectors, offering superior conductivity and resistance to corrosion. The use of gold in electronics ensures reliable performance. Furthermore, gold’s malleability allows it to be combined with other metals to create gold alloys. These gold alloy production processes result in materials with enhanced properties such as increased strength or resistance, broadening their applications in various industries.
Used in Connectors
Moving along the periodic table from zinc, the next element to explore is gold. A noble metal, gold holds a significant place in various industries. Specifically, in the realm of electronics, it is widely utilized in connectors.
The usage of gold in connectors, such as those found in high-speed data transmission cables, is paramount because it does not corrode or tarnish. Gold’s enduring physical properties, along with its excellent conductivity, make it an optimal choice for connectors where stable, long-term electrical connections are mandatory.
A gold connector ensures reliability in performance. A vital attribute considering the average consumer device contains hundreds of such connectors. Hence, the role of gold in electronics extends beyond luxury or aesthetic appeal, as its practical applications are significant and far-reaching.
The focus on gold in connectors demonstrates the versatile nature of this precious metal, further characterizing its importance beyond purely decorative or monetary value.
Gold in Electronics
Just as zinc has its significance in the world of metals, so too does gold, especially in the realm of electronics. Gold, due to its excellent conductivity and resistance to corrosion, is widely employed in the manufacture of various electronic devices.
In the field of electronics, gold is utilized in manufacturing connectors, switches, relays, and wires. Unlike other metals, gold does not tarnish or corrode, ensuring a reliable and consistent connection. Furthermore, the malleability of gold makes it an optimal choice for electronic components that require thin, yet durable coatings.
Additionally, gold is used in printed circuit boards for its excellent solderability. This aspect enables gold to be utilized in intricate and highly detailed electronic components.
Statistically, more than 10% of gold mined worldwide is used in electronics. This figure underscores the significant role of gold in the electronics industry. It is an essential material used in devices ranging from mobile phones to satellites, demonstrating its versatility and indispensability.
Gold Alloy Production
And while zinc plays a crucial role in various industries, let’s not forget the irreplaceable value of gold, especially when it comes to its usage in creating alloys.
In the world of metallurgy, gold alloy production is a significant process. The creation of gold alloys not only enhances the durability of this precious metal but also extends its application potential. For instance, 14-karat gold, a commonly used alloy in jewelry, is created by combining gold with a mixture of other metals such as copper, zinc, or silver. This combination results in a more robust and resistant material, improving the longevity of the final product.
Furthermore, gold alloys play a pivotal role in minting coins and producing dental fillings. Advances in technology have enabled the production of gold alloys with specific properties, tailored for a wide range of industrial and commercial applications. This indeed underscores the versatility and indispensable nature of gold in contemporary society.
Lead
Transitioning from its historical uses, lead plays a significant role in modern applications such as soldering due to its low melting point. However, concerns arise from its use in batteries, given the toxicity and environmental implications. Consequently, research has been directed towards lead-free alternatives in electronics, focusing on balancing functionality with sustainability.
Used in Soldering
Leaving the golden allure of gold behind, a shift towards the heavy, dull grey metal of lead marks the transition into an entirely different sphere of utility.
Lead has played a significant role in soldering applications due to its unique properties. A key component in traditional solder is lead, primarily because of its low melting point (327.5 degrees Celsius) and excellent electrical conductivity. This makes it an ideal choice for creating durable and efficient electrical connections.
In the past, lead-based solder was predominantly used for joining pieces of metal together in the electronics industry. However, the recognition of lead’s toxic nature has led to a significant decrease in its use. Despite this, lead solder still finds some application in specialized electronics where reliability under extreme conditions is a necessity.
Lead Usage in Batteries
Just as gold finds its value in the realms of electronics, another metal, lead, has made its mark in the world of power storage. Shifting the lens from gold, a look at lead in the context of batteries unravels another fascinating intersection of science and industry.
Lead-acid batteries, pioneered in 1859, represent one of the earliest rechargeable batteries. They have dominated the large-scale energy storage sector ever since. Comprising 40% of lead usage worldwide, these batteries form the backbone of automotive, industrial, and emergency power systems.
Lead provides several advantages in its usage in batteries. It has a high atomic mass and is highly conductive, which facilitates efficient energy storage and transmission. Additionally, lead’s chemical stability ensures the longevity of the battery.
However, lead’s toxicity and environmental implications necessitate careful handling. Innovations in battery technology aim to minimize lead usage and to develop alternative, lead-free solutions. One step away from gold, yet a leap into another realm of material science.
Lead-Free Alternatives in Electronics
Just as gold has its unique properties and uses, the same can be said about lead.
In the realm of electronics, lead has traditionally been a vital component. But guess what? Increasing awareness of its potential health and environmental risks led to the development of lead-free alternatives.
Specifically, numerous substitutes have emerged in the electronics industry. A prime example is tin-silver-copper solder (SAC), which is widely utilized in surface mount technology. Moreover, Indium Corporation has developed a series of lead-free solders which include bismuth-tin, bismuth-tin-silver, and tin-silver.
In addition, organotin compounds have been introduced as lead-free alternatives for electronic components. What’s more, these alternatives not only mitigate health and environmental risks but also offer improved performance in certain aspects. For instance, SAC solders have demonstrated higher drop shock resistance than traditional lead-based solders.
In conclusion, the significance of minerals in electronics is undeniable. Minerals like copper, nickel, aluminum, tin, silver, iron, manganese, zinc, gold, and lead play integral roles in various electronic devices, enhancing their functionality and durability. These minerals contribute to the conductivity, heat resistance, and other critical properties required for efficient electronic functioning.
Notwithstanding, their extraction and use continue to pose significant environmental challenges. Therefore, emphasis should be placed on sustainable mining practices to minimize adverse impacts. Responsible use of these vital minerals is a crucial step towards sustainable electronics manufacturing.



