A depositional environment is a natural or artificial layer of sedimentary material that covers an area and is created by the deposition of material from the atmosphere, water, or land. Depositional environments can be found in various places around the world. Some examples of depositional environments are beach sands, river sands, loess, tills, and gravels. A depositional environment can be a suitable substrate for plants, as it has many nutrients and is moist.
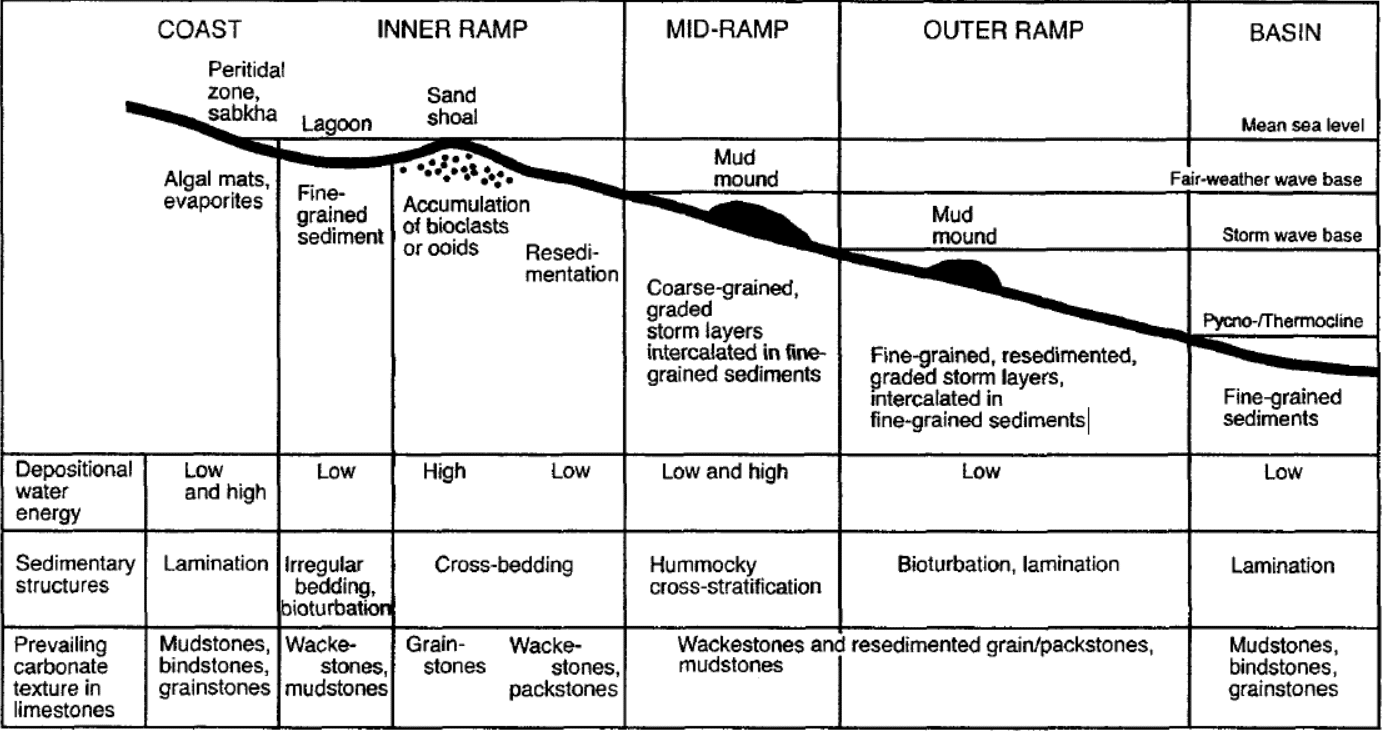
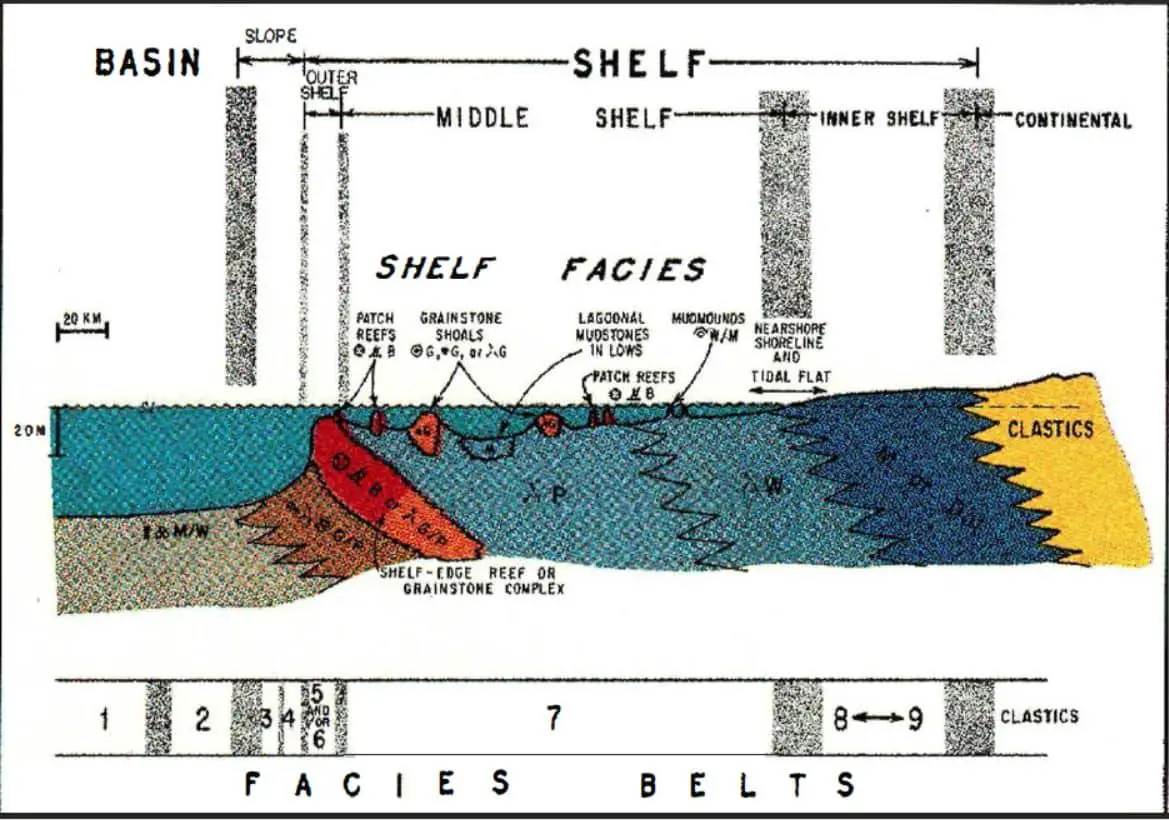
Limestones are composed primarily of the mineral calcite (CaCO3) and can be deposited in various environments. The depositional environments of limestones can be classified according to several different parameters. The most important is the nature of the sedimentary particles, which may be either clastic or nonclastic. Clastic particles are fragments of preexisting rock, whereas nonclastic particles are either chemically precipitated or derived from the breakdown of preexisting rock. The second important parameter is the energy of the depositional environment, which may be either high or low.
In general, limestones form either as a result of calcium carbonate precipitation from solution in seawater (marine limestones) or as the accumulation of calcium carbonate in lakes or swamps (non-marine limestones). The main difference between the two limestone types is their formation mode. Marine limestones form through the precipitation of calcium carbonate by organisms that live in seawater.
Limestone deposited in a shallow marine environment is likely more fossiliferous than one deposited in a deep marine environment. This is because shallow marine environments are more conducive to preserving organisms than deep marine environments. In addition, limestone deposited in a shallow marine environment is likely to be more bioturbated than one deposited in a deep marine environment.
Composition of Limestone
A rock is considered to be limestone if it contains at least 50% calcite (calcium carbonate). All limestones contain at least a trace amount of other elements. W Cave action or currents can transport some amounts of feldspar, clay, or quartz mineral particles to the location. Chert, siderite, pyrite, and other mineral fragments can form as a result of chemical reactions the limestone undergoes. When limestone comes into contact with a cold solution of 5% hydrochloric acid, it fizzes, a characteristic frequently used to identify rocks.
Oolitic limestone depositional environment
Oolitic limestones are among the most common sedimentary rocks in the world. They are formed in various environments, including marine, lacustrine, and fluvial settings. The material is mostly carbonate, but iron oxides or amorphous silica also occur. Oolitic limestones are characterized by their small ovoid grains composed of calcium carbonate. These grains are formed by oögenesis, in which small particles of calcium carbonate are precipitated around a nucleus, such as a grain of sand.
A seed of some kind, possibly a piece of shell, is where formation starts. The strong currents that wash over the seed causes it to accumulate a layer of calcite that has chemically precipitated from the super-saturated water. So the environment is usually marine but close to a shallow beach to have wave action and grains for the formation. This is usually the case in a small closed basin separated from the wide sea where evaporation causes the oversaturation or in tropical areas, where oversaturation is also often found. Only an infinite amount of oolites are used in this procedure. Enormous dunes (mega-ripples) are where the oolites are often located; if a human were there, they could pick them up with their hands.
The concentric layers are formed by the oolites being simultaneously exposed to take up a concentric layer and then submerged to set the layer. The subsequent exposure subsequently creates a new layer. In a lower intertidal or subtidal environment, they are frequently connected to areas of significant tidal activity. Oolites typically form in warm, shallow waters with high levels of dissolved calcium carbonate.
One may see the seed that started the oolite in the center and the concentric layers by which it was constructed when some of the shattered oolite samples are examined. The oolite resembles a small pearl in the unbroken specimens. As the ooliths settle to the bottom of the water column, they are slowly cemented together by calcium carbonate to form a solid rock.
After the formation process, the oolites are transported to the shoreline by the waves and currents. The oolites are then deposited on the beach. Clear calcite precipitated between the oolites by groundwater after deposition serves as the spar cement. It resembles a translucent or light gray glue that holds the oolites together.
Carbonates of various kinds are frequently found in tectonic stability, tropical temperatures, stable, shallow water environments connected to the continental shelf or epeiric (epicontinental) seas, and no mountain formation. The oolitic limestone depositional environment is created by the interplay of two main processes: physical processes, such as wave action and currents, and biological processes, such as the activity of filter-feeding organisms. Oolitic limestone is typically found in shallow marine environments where there is a high amount of wave activity. Oolitic limestone is often used in construction and is a popular stone for carving.
Fossiliferous limestone depositional environment
Fossiliferous limestone refers to limestone that has a high concentration of visible fossils. Fossiliferous limestone is created by accumulating shell, algal, coral, fecal, and other organic waste. According to the Folk classification of sedimentary rocks, biosparite limestone is the name for fossiliferous limestone. Fossiliferous limestone is included in the group of rocks known as lagerstätte.
Most limestones are created in warm, still, and clear shallow waters. In such kinds of environments, organisms that produce calcium carbonate shells and skeletons may thrive and easily get the required ingredients from ocean water. These organisms leave behind the shell and skeletal remnants that can ultimately lithify into limestone once they die. Furthermore, their waste products contribute to the silt pile. Sometimes currents, organisms, dissolution, or recrystallization can obliterate biological origin-related evidence.
Fossiliferous limestone harbors information about the habitats of the organisms and the environment of deposition. Paleontologists frequently determine the geological age of the rock by examining the fossils. These rocks include both macroscopic and microscopic fossils. Crinoid stems, gastropods, brachiopods, and other hard-shelled mollusk remnants are examples of macroscopic fossils. The sole presumed evidence of bioactivity retained in limestone can sometimes be found in microfossils, for instance, siliceous diatom shells in the deposition.
On Earth today, there are several situations where limestone may occur. Most are located between 30 ° north and 30 ° south latitude in shallow oceanic regions. The Indian Ocean, Caribbean Sea, Persian Gulf, Pacific Ocean islands, Gulf of Mexico, and Indonesian archipelago are all experiencing limestone formation. Massive volumes of calcium carbonate skeleton debris and fecal matter are produced in these locations by an abundance of corals, algae, shellfish, and other species covering the platform entirely. A significant deposit of calcium carbonate sediment produced turns into limestone.
Evaporative (Cave) limestone depositional environment
Evaporation can also form limestone. Limestone that developed by evaporation includes stalagmites, stalactites, and other cave formations (often referred to as “speleothems”). Through cracks or other pore areas in the cave ceiling, water drops flowing down from above enter the cave. Before dropping to the cave floor, the droplets may evaporate, leaving the calcium carbonate.
Any dissolved calcium carbonate is precipitated when the water evaporates. This evaporative process can cause calcium carbonate crystals in the form of icicles to build up on the cave ceiling over time. Stalactites are pillar-like structures formed as a result of the process. If the drops land on the ground and evaporate, stalagmites may gradually rise from the cave’s floor.
Travertine, a chemical sedimentary rock, is the name given to the limestone that makes up these cave formations. A limestone rock called “tufa” is created in an arid region by evaporation near a hot spring or on a lake’s shore.
Micritic limestone depositional environment
Micritic limestone is a type of limestone that is composed of very small, fine-grained particles. It is usually formed from the fossilization of marine organisms such as plankton. The mineral calcite and lime mud make up the micritic limestone. In clastics, micrite is the shale rock equivalent of clay. It is deposited as tiny aragonite needles but later changed to calcite, and the calcite was subsequently cemented to create the rock. One of the most typical carbonate rocks is micrite, which makes up most of the limestone. It is deposited in typically calm water, much like clay (shale), and may be found everywhere such conditions occur.
Micritic limestone usually forms in a quiet, deep water environment where calcium carbonate precipitation exceeds the dissolution rate. The lack of wave and current activity allows the formation of very small (microscopic) calcium carbonate skeletons, which are then deposited on the sea floor. Over time, these skeletons can build up to form a thick layer of micritic limestone.
Micritic limestone has a conchoidal fracture and is a solid, homogeneous, fine-grained rock. This micrite exhibits the characteristics of pure rock since it has no discernible structure at all. One thing to remember is that micrites are often light gray or brown, although contamination with other materials is not unusual, which brings us to the hypothesis that applies to sedimentary rocks: “Never Trust Color” when it comes to classifying them. You’ll be misled by it more often than not.
Bioclastic limestone depositional environment
A bioclastic limestone is a sedimentary rock that forms in shallow, warm seas with plenty of marine life and a high concentration of biogenic or organic particles. It’s composed of the skeletal remains of marine organisms, such as coral, mollusks, and echinoderms. The skeletal fragments are called “bioclasts. These fragments can be either organic or inorganic.
Bioclastic limestone comprises two parts: the bioclasts, or fossil debris, and the matrix, or calcium carbonate. The bioclasts can be any fossil, such as shells, corals, or stromatolites. Most bioclastic limestone depositional environments are thought to have been formed during the Late Devonian period. They are known as tropical bioclastic limestones in the warm seas of the tropics at low latitudes and temperate bioclastic limestones in the colder waters at mid to high latitudes.
Depositional environment of chalk
Chalk refers to limestone that is formed from the buildup of calcareous shell fragments left behind by small aquatic organisms such as foraminifera. It can also develop from some marine algae’s calcareous remnants. Chalk is an extremely fine-grained, brittle rock that may be readily crumbled or crushed. It often has a color white or light gray.
The chalk formed in various depositional environments during the Cretaceous Period, 145 and 66 million years ago, including deep-water environments, such as the Western Interior Seaway in North America, and shallow-water environments, such as the Paris Basin in Europe. The environment that this chalk formed in was very different from the modern-day environment. The sea was shallower, and the water temperature was warmer. The chalk was formed from the remains of microscopic marine organisms called coccolithophores.
During the Cretaceous era, natural chalk occurred in deep sea conditions and consisted of a gradual accumulation of very small calcite plates. The Cretaceous was a time of global warming, which caused the Earth’s oceans to rise significantly. This rise in sea level resulted in chalk deposition in shallow marine environments. The chalk deposited during this time is well preserved and provides a wealth of information about the Cretaceous environment.
Chalk is widespread across Western Europe, where deposits underlie areas of France where chalk deposits reach the sea, such as the Dover cliffs on the Kent coast of the English Channel; high cliffs are frequently visible. Chalk is mined for application in agriculture to raise pH in acidic soils and make bricks, quicklime, and builder’s putty in the industry. It is also employed as “blackboard chalk” for drawing and writing on different surfaces. Gypsum or other carbonate-based minerals can also be used to make chalk for writing and drawing.
References
Blyth Cain, J. D. (1968). Aspects of the depositional environment and palaeoecology of crinoidal limestones. Scottish Journal of Geology, 4(3), 191-208.
Geological society—Limestones. (n.d.). Retrieved January 10, 2023, from https://www.geolsoc.org.uk/ks3/gsl/education/resources/rockcycle/page3524.html
Kilic, Ö. Z. E. N. (2013). Impact of physical properties and chemical composition of limestone on decomposition activation energy. Asian Journal of Chemistry, 25(14).
Micrite—An overview | ScienceDirect topics. (n.d.). Retrieved January 10, 2023, from https://www.sciencedirect.com/topics/earth-and-planetary-sciences/micrite
Oolitic limestone. (n.d.). Retrieved January 10, 2023, from https://csmgeo.csm.jmu.edu/geollab/fichter/sedrx/rocks/oospar1.html#:~:text=Oolites%20form%20today%20in%20warm,sort%2C%20perhaps%20a%20shell%20fragment.
Sandstones. (n.d.). Retrieved January 10, 2023, from https://sites.pitt.edu/~cejones/GeoImages/5SedimentaryRocks/Carbonates/Bioclastic.html
Science, G. (2019, May 9). Chalk | properties, composition, formation, and uses. Geology Science. https://geologyscience.com/rocks/sedimentary-rocks/chalk/
Staff, A. E. E. (2020, June 21). Limestone—Formation, composition, types, and uses | earth eclipse. https://eartheclipse.com/science/geology/limestone.html
Zhang, W., & Lv, C. (2020). Effects of mineral content on limestone properties with exposure to different temperatures. Journal of Petroleum Science and Engineering, 188, 106941.



